Exercise #1
a) Consider the following thermodynamic cycle:
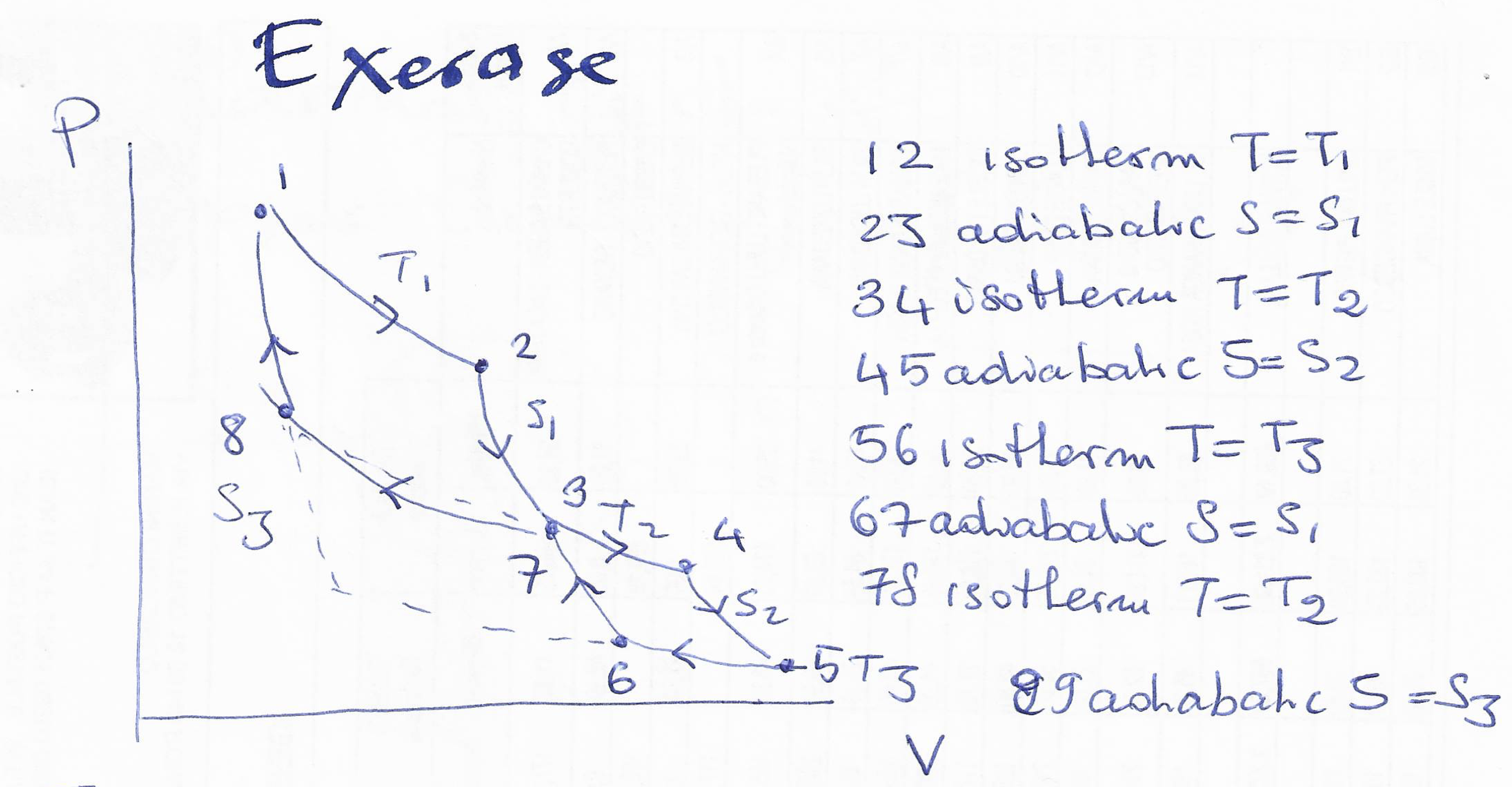
-
Draw the cycle in the T-S plane.
-
Calculate the total work exerted by the system (W).
-
Calculate the total heat exchanged by the system (Q).
-
Calculate the efficiency $\eta={W}/{Q_{abs}}$ where $Q_{abs}$ is the absorbed heat.
b) Include the fluctuation of number of particle in the description of stability and thermodynamic fluctuations. Perform the calculations in the variables T,V,N and S,P,N.
-
Are fluctuations in these variables uncorrelated?
c) Consider a system of $N$ distinguishable quantum systems having two non-degenerate levels at energy $E_0$ and $E_1$. They are thermalized at temperature $T$.
-
Calculate the internal energy U(T,N).
-
Calculate the entropy S(T,N).
-
Eliminate temperature to get S(U,N) and discuss the results.